Introduction to Residual Gas Analyzers
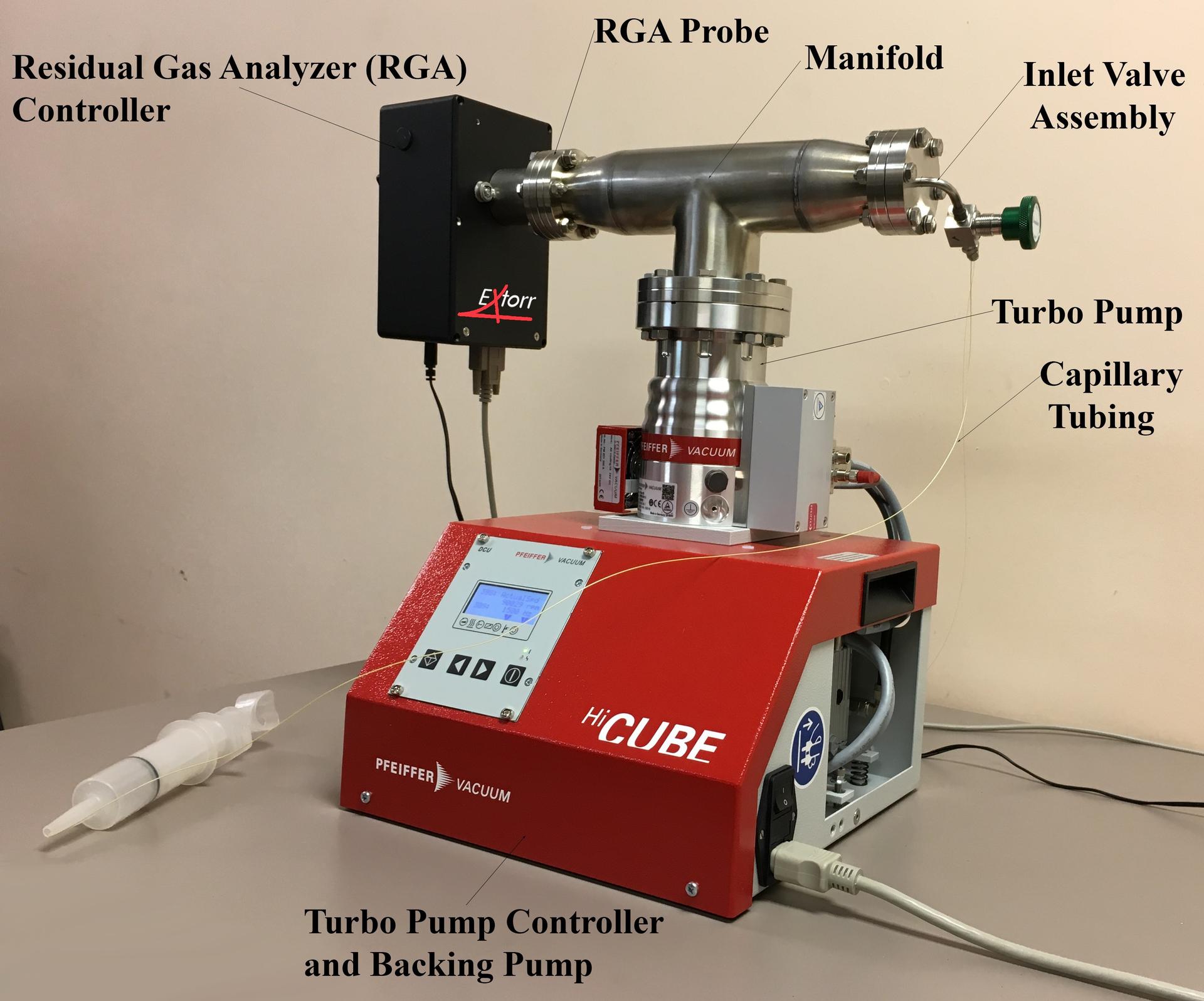
A residual gas analyzer (RGA) is a mass spectrometer that can help determine the composition of the substances remaining in an imperfect vacuum chamber. A typical modern residual gas analyzer ionizes some of the gas in the vacuum chamber with electrons from a filament. The ions are then directed into a quadrupole mass filter that uses electric fields to pass only the ions of a particular mass. Finally, the flowing charge of ions are detected as a current. By varying the electric fields in the mass filter and recording the resultant current to the detector, a mass spectrum is obtained. The sensor probe, consisting of the ionizer, quadrupole mass filter, detector and electrical vacuum feedthrough is installed into the vacuum chamber. The electronics package is connected to the atmosphere side of the electrical feedthrough and a computer.
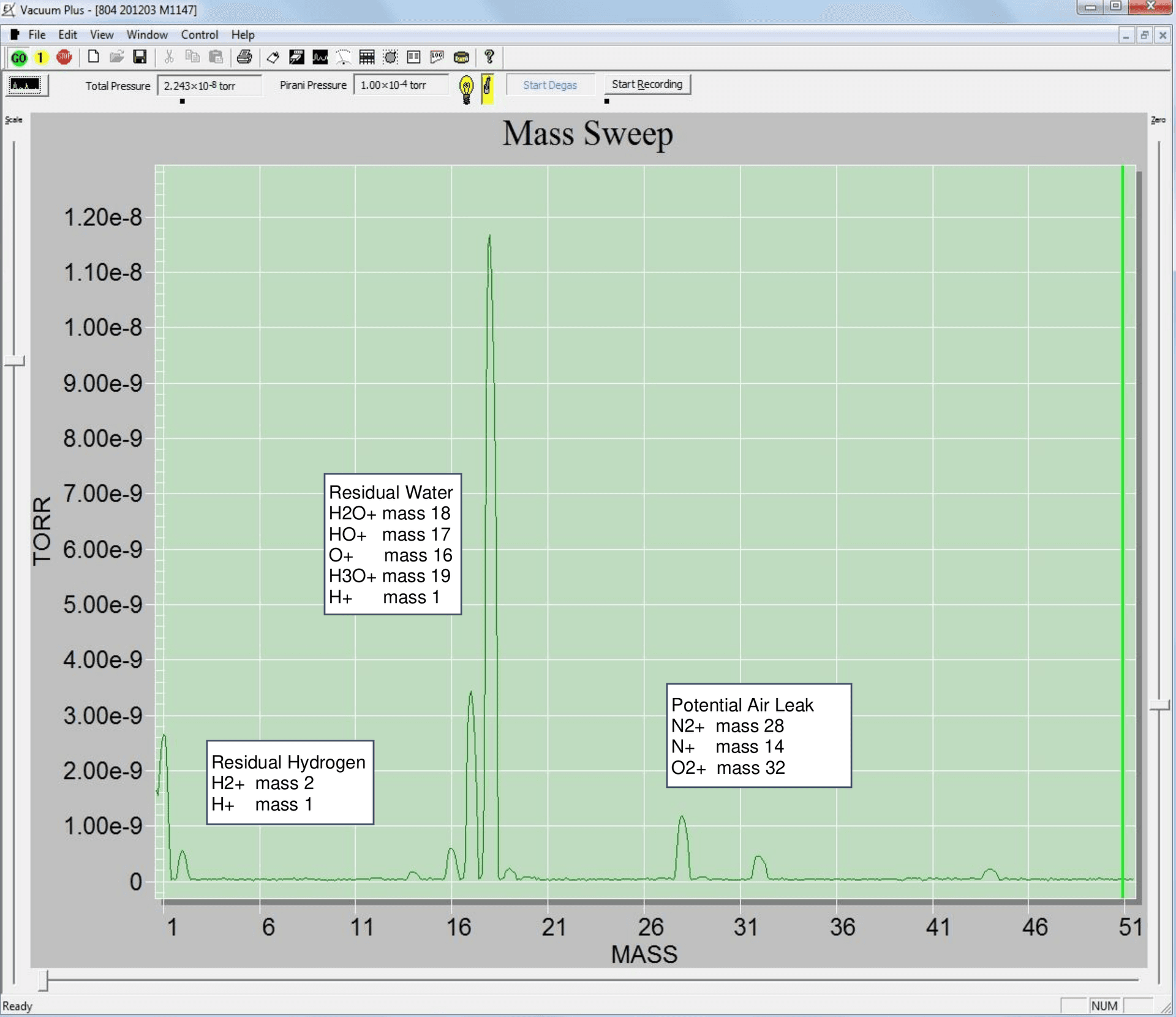
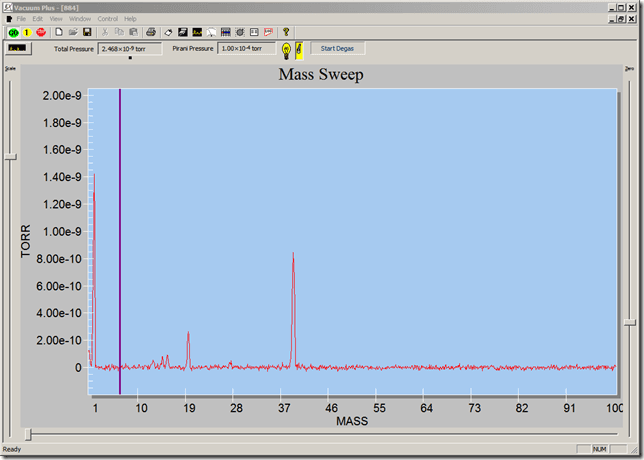
For example, an RGA is installed onto a vacuum chamber and produces the following spectrum at 2.2 x 10-8 torr:
Water is usually the limiting residual in an unbaked high vacuum chamber. Most H2O molecules consists of a common oxygen isotope 16O atom (8 neutrons and 8 protons) combined with 2 Hydrogen atoms (1 proton each) and thus has a mass of 18 amu (or 18 Da). As the ions are produced in the RGA ionizer, along with the parent H2O+, some ofthe molecules fragment into HO+, H+, O+, and so on, adding to the peaks at their respective amu. Referring to the annotations in figure 1, the peak at 1 amu is from both the H2O and H2. The peaks can give qualitative insight to the contents of the chamber, but interpretation is required, and quantitative analysis is not trivial.
Residual Gas analyzers and mass spectrometers do not measure partial pressures. They measure ion current at a detector for each atomic mass number. The RGA uses electron impact to ionize molecules in the ionization volume of the ion source. Every molecule has its own unique probability for ionization (the ionization cross section). The RGA will have a different sensitivity for each gas species. For example, the RGA measures a current at the detector for mass 28, It could be nitrogen, carbon monoxide or other molecule, the mass spectrometer cannot know this. It would not be correct to convert this ion current to partial pressure without knowing the gas species, but since most gasses are within a factor of 2 sensitivity of nitrogen, it is customary to use nitrogen calibration for all mass numbers and report partial and total pressures this way for an approximate partial pressure.
Mean free path effects
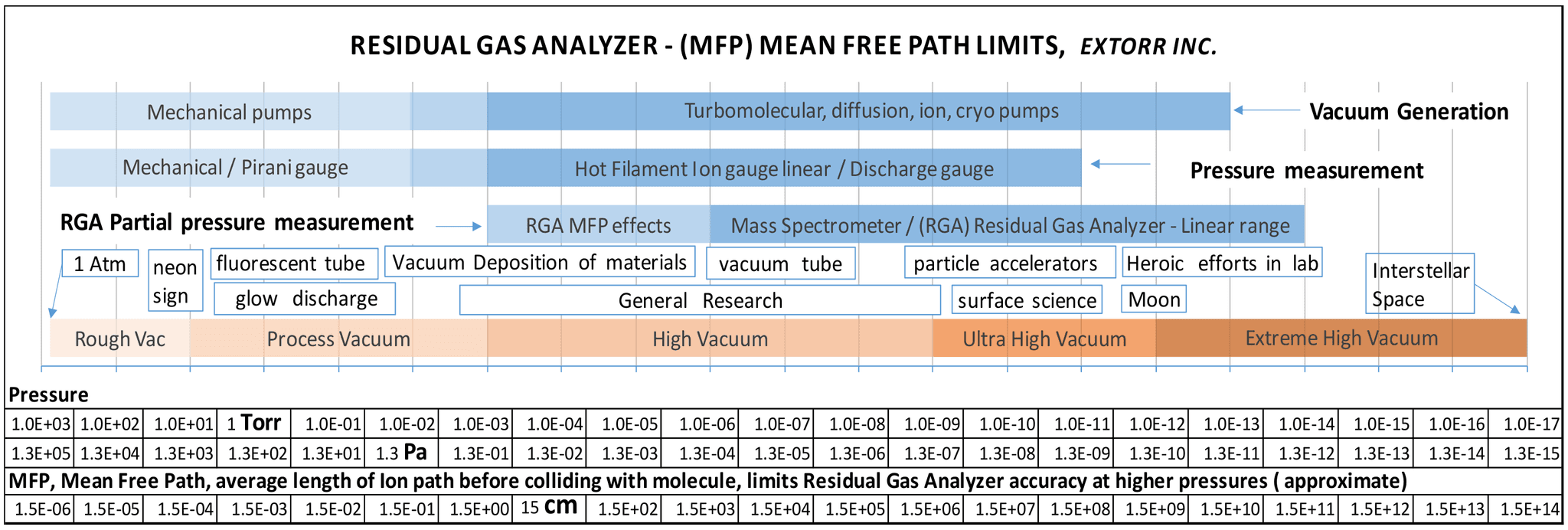
The most important concept in understanding how to use a residual gas analyzer is the mean free path of a gas ion/molecule. The RGA is used in a vacuum chamber where the pressure is low enough so that the ions are unlikely to collide with other gas molecules before they are filtered and detected. At pressures above 1 x 10-6 torr, when the mean free path becomes smaller, some ions will collide with gas molecules before reaching the detector, reducing the peak heights. Some chemistry may also occur, further complicating the analysis.
The above diagram gives approximate mean free paths for ion-molecule collisions. The size, speed, charge, etc. of the molecules and ions will cause variations. It might seem that the dimensions of the probe are on the order of 12 cm., but the quadrupole uses a high frequency oscillating field to sort the ions, increasing the path length of an ion through the quadrupole to the detector many times that.
If the RGA is to be used at pressures above 1x 10-6 torr, there are 2 choices
- Use a second chamber with the RGA and a high vacuum pump, connected through a flow restrictor to the higher pressure gas sample of interest, reducing the pressure to the linear range at the RGA.
- The RGA will work up to 3 x 10-3 torr for qualitative work and, with careful calibration, for quantitative results. The exposure of the RGA to high pressures of chemically active gasses for prolonged periods of time will deteriorate the ionizer, filament and quadrupole mass filter. Even water vapor will take it’s toll on the hot internal surfaces of the ionizer. Use for leak checking and periodic residual gas analysis.
Since the RGA used here contains a separate internal ion gauge with a path of about 1 cm to the detector, the total pressure displayed is correct, even at 3 x 10-3 torr. This is a big help with estimating quantities, since the partial pressure peaks are compressed.
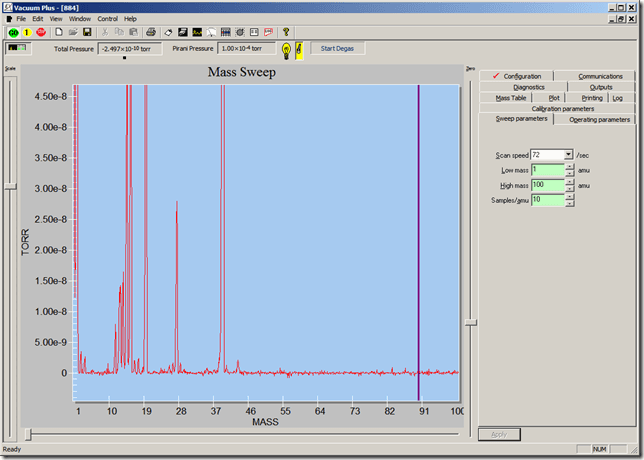
Air Spectra at increasing pressures, showing the MFP effects:
Very long mean free path, partial pressures are good: Air at 2 x 10-6 Torr
Mean free paths of ions begin to show increasing effects, partial pressures will need corrected for quantitative work:
Air at 2 x 10-5
Air at 6 x 10-5
Air at 1 x 10-4
Air at 3 x 10-4
Air at 1 x 10-3
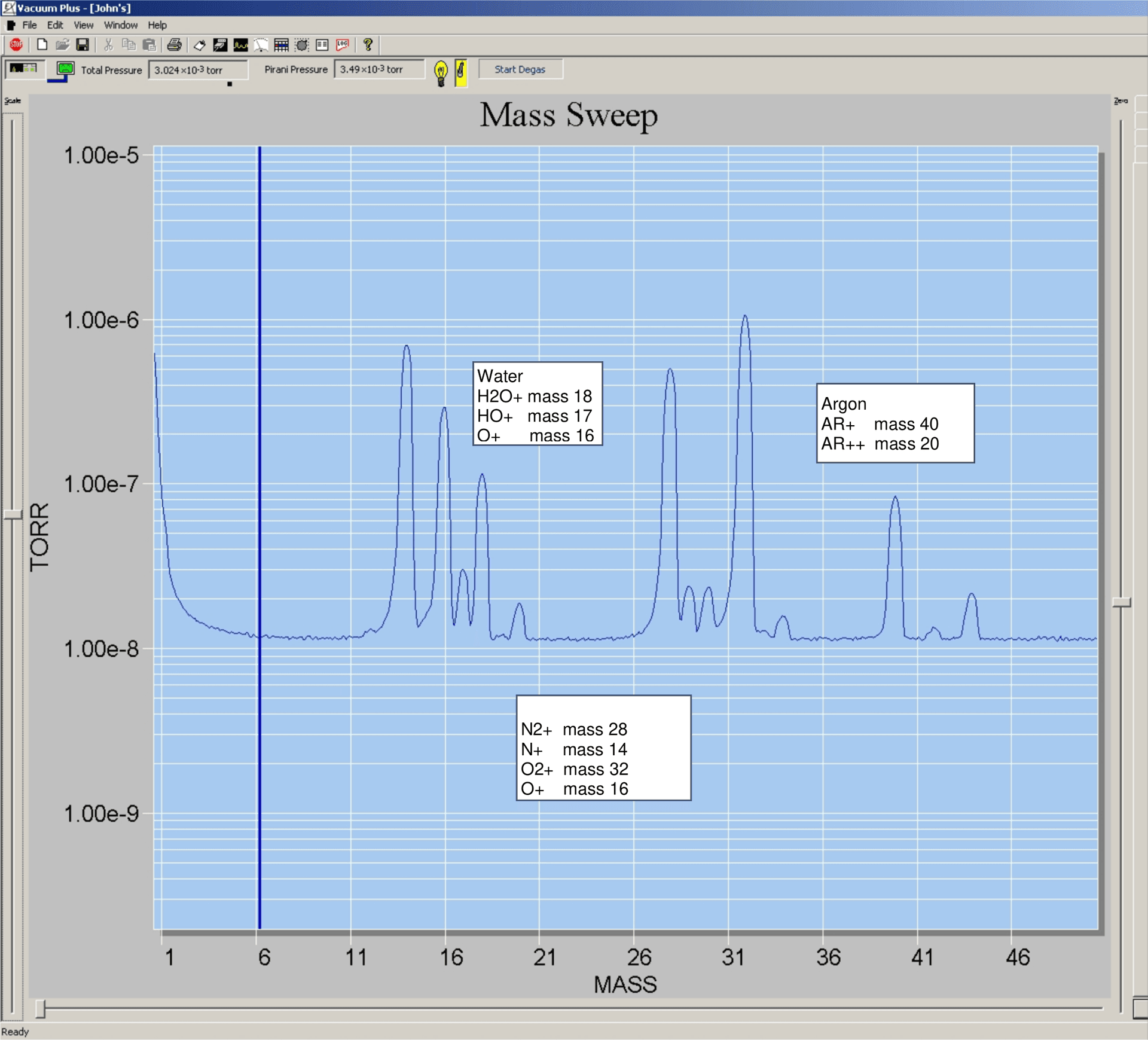
Air at 3 x 10-3
Air at 5 x 10-3
Air at 1 x 10-2
Air at 3 x 10-2
The benefit of an electron multiplier
If you get down into the UHV range, the electron multiplier can be beneficial. The sensitivity is improved and the scanning speed can be increased for the same signal to noise ratio.
Here is an RGA in a small chamber at 2.5 x 10-9 torr with a getter that has been baked and evacuated. You can see the hydrogen and a small amount of methane at mass 2, 15, 16 from the getter. At 20 and 40 you can see Ar++ and Ar+, that are not pumped well by the getter. This scan will take several minutes, 500 samples at 10/second. You can barely see the CO peak at 28 amu, but this is an exceptionally clean system: